My colleagues and I had another paper published last week. From the title page, click on the image to read the paper.

I worked with Henry Lin at the NM VA Healthcare System, the UNM CTSC, and UNM Sleep disorders clinic to study whether increased exposure to the stress hormone norepinephrine would result in a dysbiosis (defined as unhealthy changes in the makeup of the microbiota) in patients with obstructive sleep apnea.
We chose to look at obstructive sleep apnea (OSA) because it is the archetype of disordered sleep occurring in 10-20% of the population. And sleep disturbance is associated with myriad health problems and early mortality. When we designed this study, an increasing number of studies had shown a possible role for the gut microbiota in the health problems linked with OSA – obesity, diabetes, cardiovascular disease – but not with OSA itself.
One mechanism, that had not been explored until our study, was increased growth and virulence of pathogenic bacteria after exposure to stress catecholamines. Mark Lyte was instrumental in highlighting this harmful effect of stress hormones on the gut microbiota. He and his colleagues showed increased bacterial growth from norepinephrine in animal and in vitro models, occurring because catecholamines impair the host sequestration of iron by iron-binding proteins, such as transferrin.

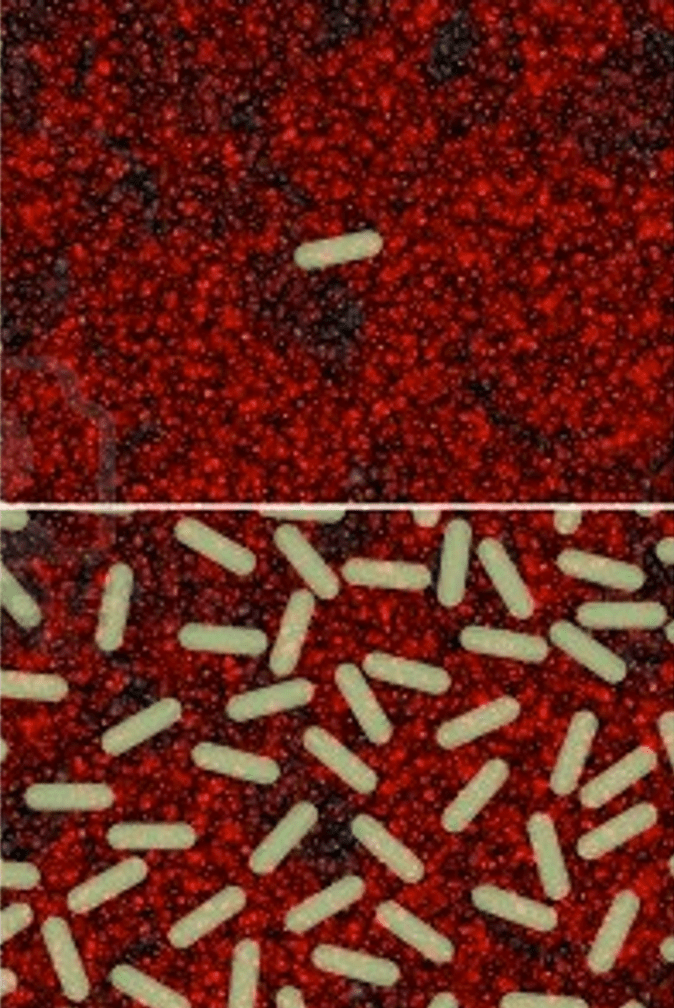
Three years ago, in a separate study, our group showed that norepinephrine stimulates the growth of Desulfovibrio vulgaris. DSV is a gram negative pathobiont (potential pathogen):
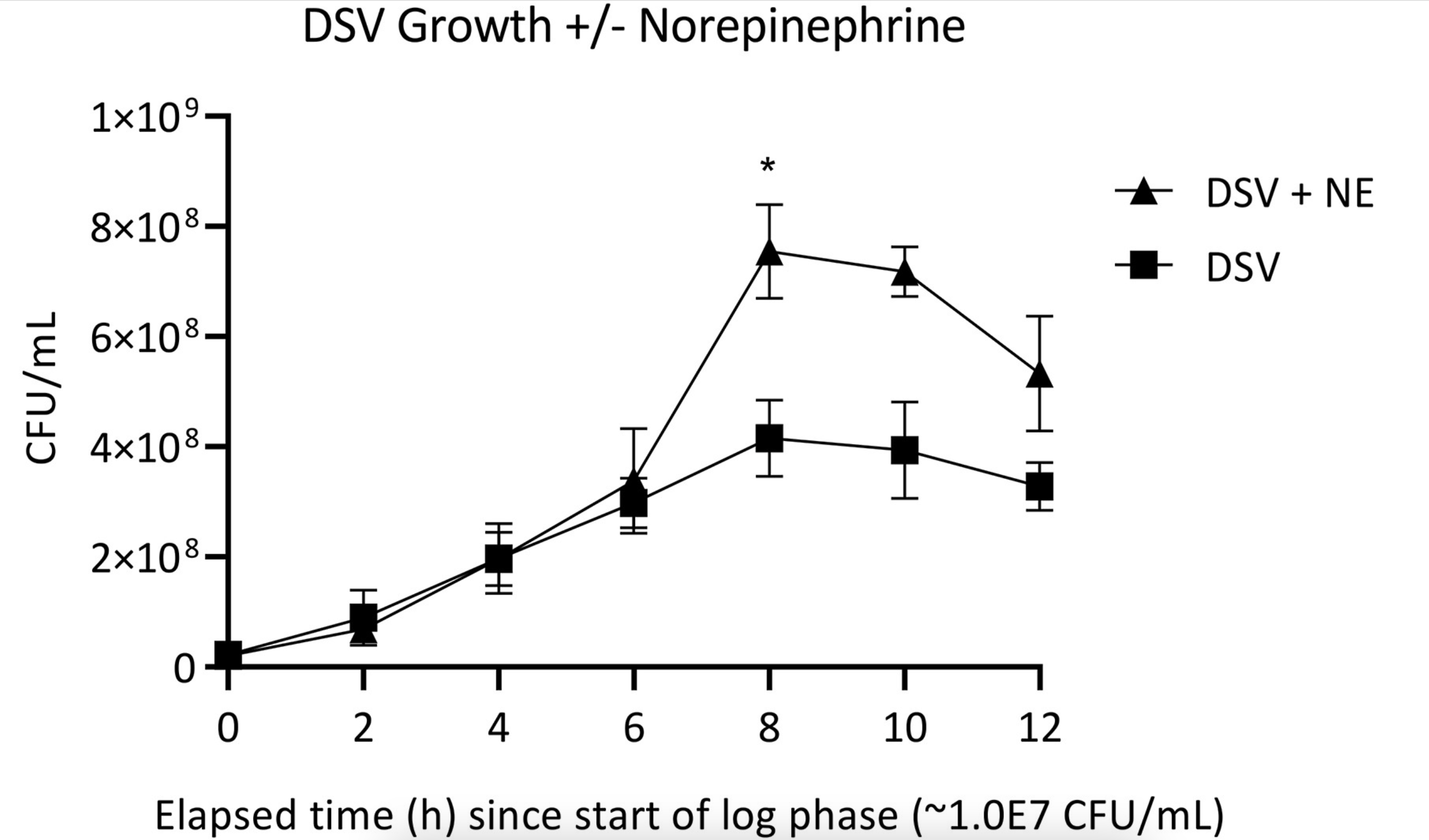
Sleep apnea is associated with increased exposure to norepinephrine. NE is what wakes up patients who have stopped breathing from upper airway obstruction. We reasoned that obstructive sleep apnea patients would have a gut microbiota dysbiosis and possibly an overgrowth of E. coli that are particularly sensitive to the growth stimulating effects of norepinephrine. This illustrates a tradeoff around stress and stress hormones – these are necessary to prepare he body for a challenge, but they may also impair certain antibacterial iron-related defenses.
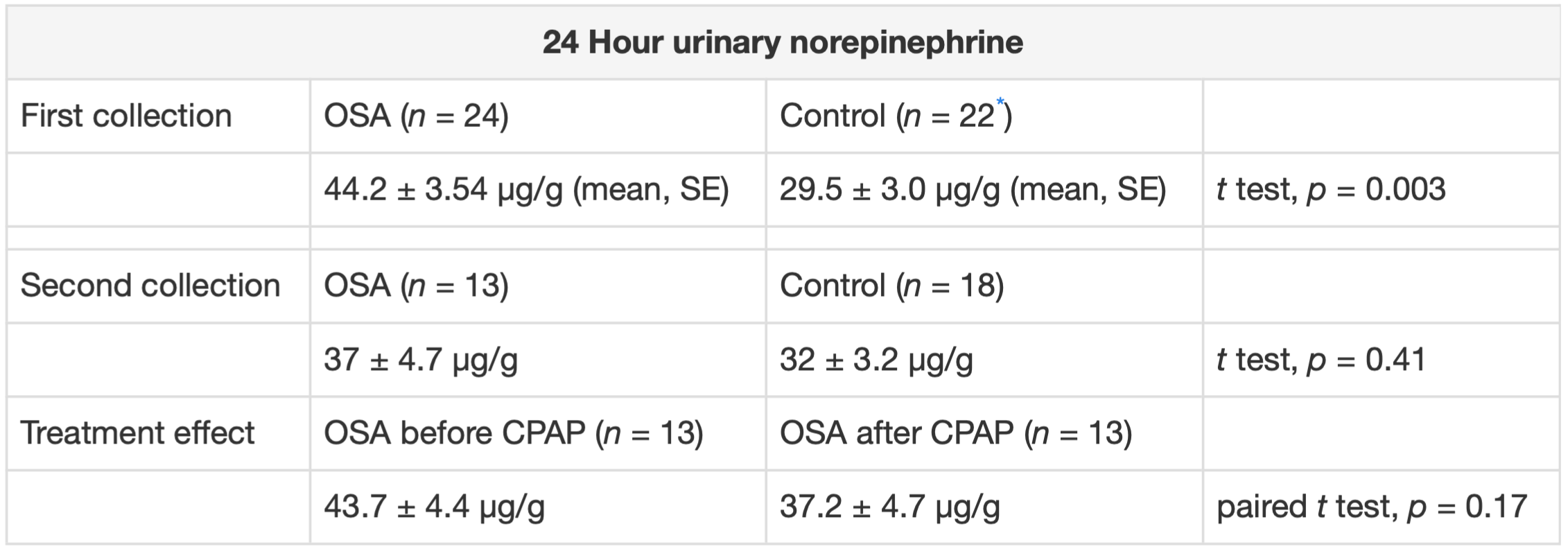
In our study, we found that urinary norepinephrine was higher in untreated OSA patients as compared to controls. Treated OSA patients had similar NE levels to controls.
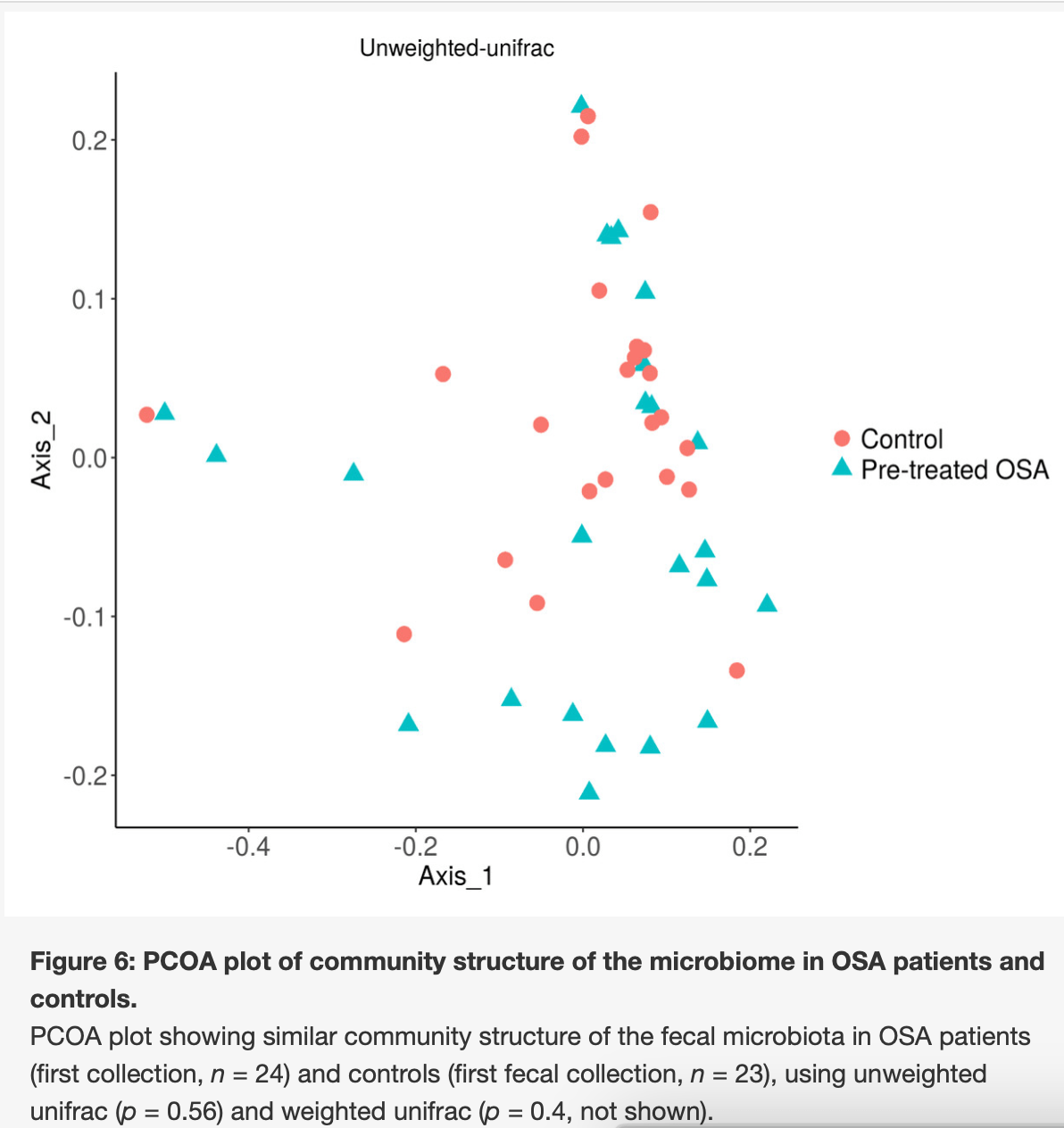
The overall structure of the gut microbiota was similar in obstructive sleep apnea patients and controls.
But we did follow patients and controls longitudinally. We found that within subject changes in norepinephrine – which was measured in a 24 urine collection – showed a positive linear relationship with the abundance of gram negative enterobacteria (the group containing E. coli.)
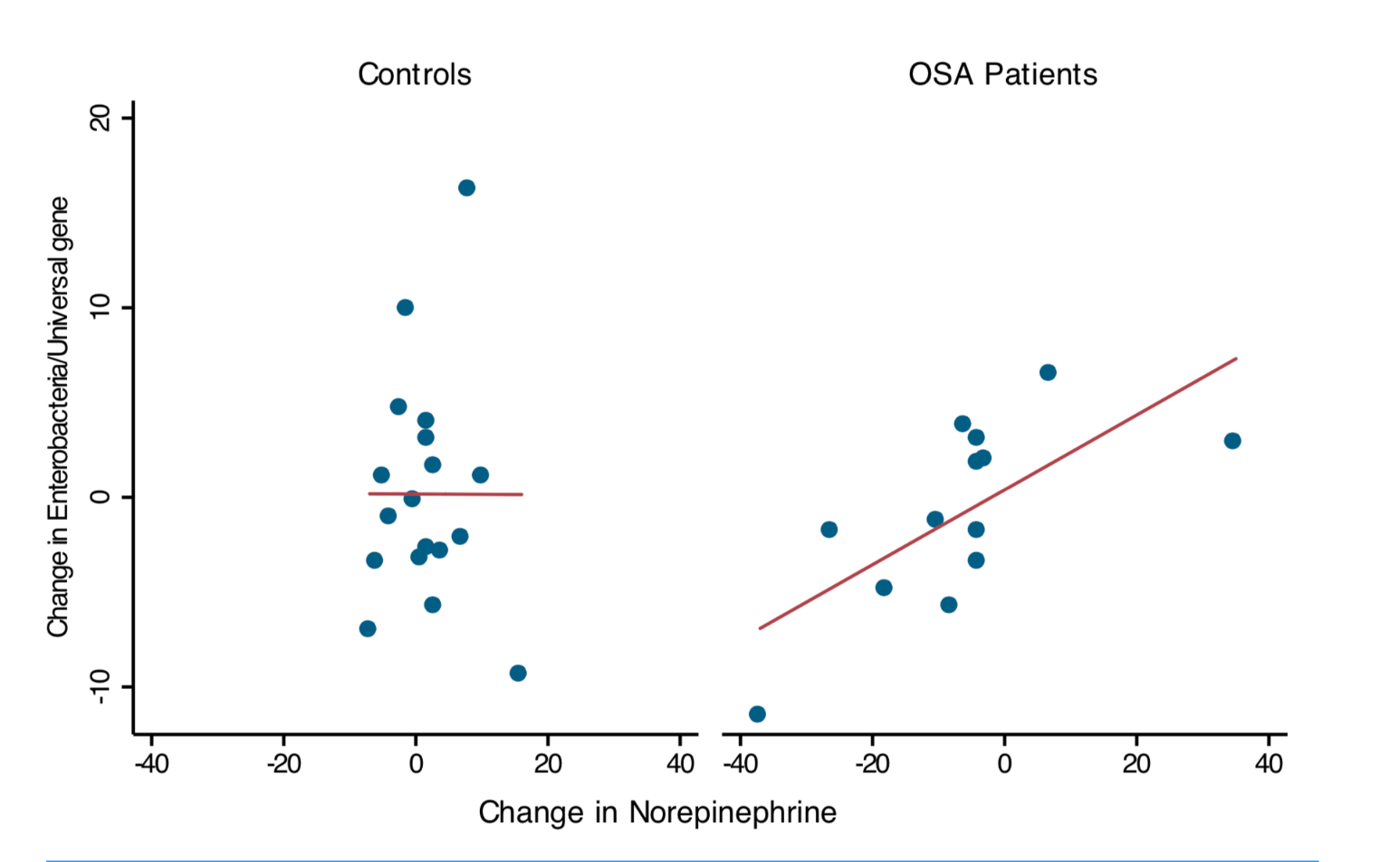
This was not linked to changes in intestinal inflammation or to the severity of the sleep apnea, as measured by the apnea/hypopnea index. However, our study is the first to translate the robust preclinical literature showing adverse effects of catecholamines on gut pathogens to OSA, a common clinical condition typified by elevated catecholamines. We found a within-subject effect when we looked at longitudinal changes in enterobacteria measured by qPCR and longitudinal changes in norepinephrine. These changes were absent in controls.
Bottom line: stress hormones may play a role in the abundance of enterobacteria in sleep apnea patients, although this relationship does not mean that sleep apnea patients gut microbiota is different overall.
The abstract is here:
Patients with obstructive sleep apnea (OSA) have increased mortality from chronic inflammatory and cardiovascular diseases. Excess catecholamine exposure contributes to the disease associations of OSA, but the underlying mechanism is unknown. This study tested the hypothesis that increased catecholamine exposure is associated with Enterobacteriaceae abundance in OSA. We compared urinary norepinephrine and the fecal microbiota in 24 patients with OSA and 23 controls. Urinary norepinephrine was elevated in OSA patients, consistent with increased sympathetic activation in those patients. OSA patients did not show changes in the community structure of the microbiome or in Enterobacteriaceae abundance compared to controls. Longitudinal changes in Enterobacteriaceae abundance in OSA patients were significantly associated with within-subject changes in norepinephrine, but this association was absent in controls. These results provide a preliminary association between norepinephrine exposure and Enterobacteriaceae in patients with disordered sleep.
Read the open-access paper here: Catecholamine exposure and the gut microbiota in obstructive sleep apnea
Categories: Uncategorized
Joe Alcock
Emergency Physician, Educator, Researcher, interested in the microbiome, evolution, and medicine
Doesn’t higher CO2 stimulate breathing? Does it occur via NE?
Interesting. You are busy publishing these days!
Cynthia
Thanks, Cynthia. Absolutely. A combination of hypoxia and hypercarbia stimulate breathing because of NE in sleep apnea. In sleep apnea, profound hypoxia and hypercarbia cause awakenings triggered by NE release from the adrenal gland. Interestingly, people debate whether mild OSA needs to be treated. Like the respiratory depression that occurs in normal sleep that causes relative hypoxia even in the absence of OSA, mild OSA may not be associated with severe health outcomes.