Thymosin α1 is an immune modulating drug that was recently the subject of a large scale multi-center randomized clinical trial in China. Thymosin α1 modulates T-cell function and improves antigen presentation among other things. It had shown promise in multiple single center trials. The most recent by Wu et al. published in the BMJ, was performed in 22 centers in China. It involved 1106 adults with sepsis (by Sepsis-3 criteria) who were randomly assigned in a 1:1 ratio to receive thymosin α1 (n=552) or placebo (n=554). Mortality was unchanged in the intervention group and control (23.4% thymosin α1 vs. 24.1% placebo).
Careful followers of this blog know that I am very critical of immune modulating drugs that block immune function (typically with anti-inflammatory mechanisms) in sepsis, because of the remarkably poor track record of that approach. For instance John Marshall found more than 100 randomized controlled trials of immune modulating agents that were all ultimately negative. At around the same time, Opal et al. published this summary of state of sepsis trials in Critical Care 2014 (the interventions without arrows were dead ends):
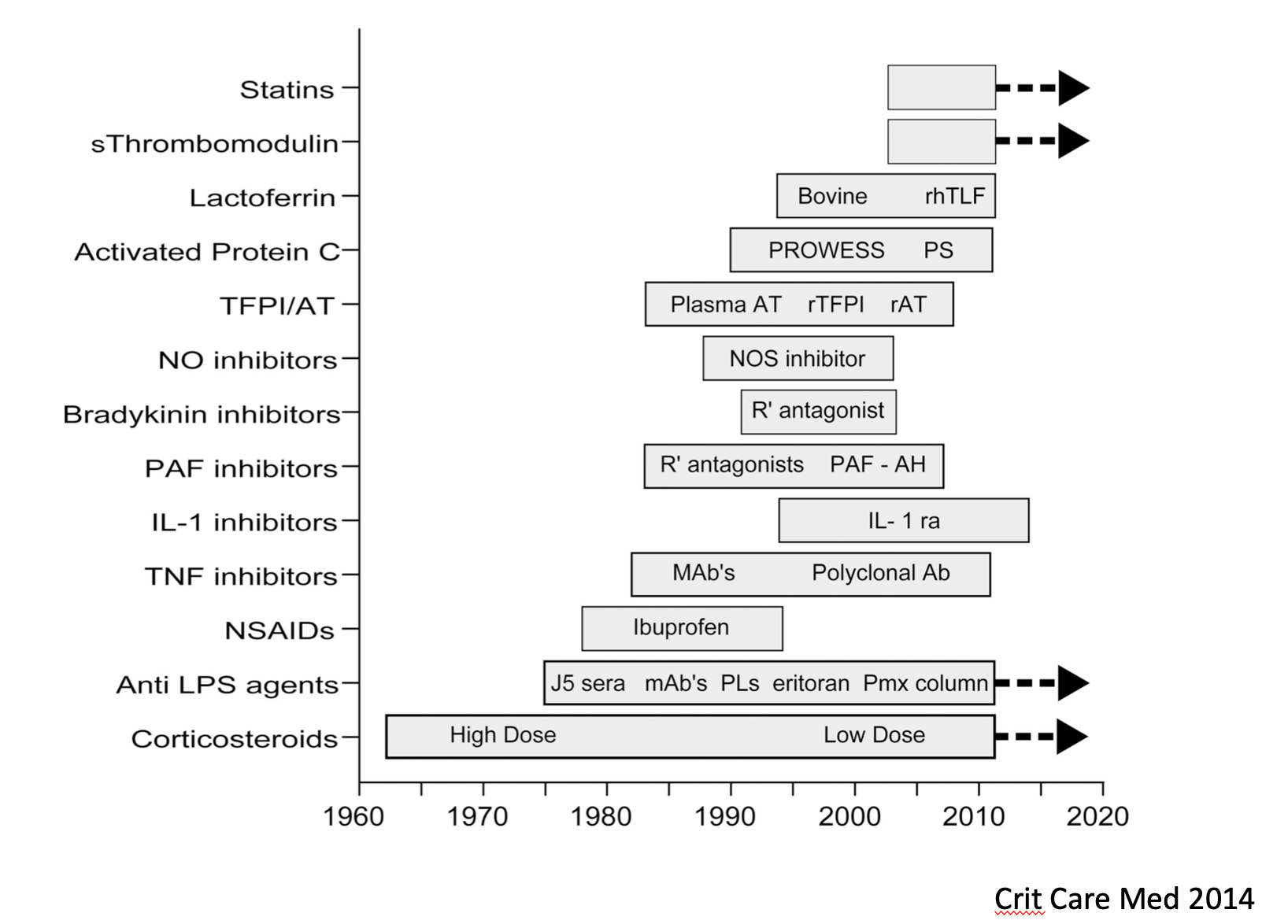
At that time, four possible strategies (shown with arrows) were under active study – statins, polymyxin B hemoperfusion, low dose corticosteroids, and soluble thrombomodulin. The best designed trials of these approaches all proved negative in the intervening years. (Note – people still argue about corticosteroids, but see in particular the ADRENAL trial. The recently published REMAP-CAP trial also argues against corticosteroids for patients with severe pneumonia. See also my previous entry, Are we prescribing steroids too much?)
With Wu et al 2025, thymosin α1 joins those failed strategies. They concluded “this trial found no clear evidence to suggest that thymosin α1 decreases 28 day all cause mortality in adults with sepsis.” thymosin α1’s immune modulating effects are complex, boosting T cell immunity and antigen presentation, reducing certain pro-inflammatory cytokines such as TNF-α, and increasing others, such as interferon α and γ. Boosting immunity might work sometimes in sepsis. Notably Hotchkiss and Drewry showed that increasing body temperature in hypothermic sepsis patients improves survival (which is also in agreement with the view that fever is adaptive in sepsis). To date, though, no drug that specifically targets immunity in sepsis has consistently improved survival.
Why does this matter? It matters because these trials collectively argue against the dysregulation hypothesis of sepsis.
As I wrote in my 2018 paper, “The Emperor Has No Clothes? Searching for Dysregulation in Sepsis:”
“The preponderance of evidence indicates that sepsis survival is not improved by blocking one or many immune pathways. Similarly, improvements in sepsis mortality are resistant to modification by normalizing one or many physiologic parameters simultaneously. The vast majority of interventions are either ineffective or harmful. As a predictive heuristic, the dysregulation hypothesis has a repeated track record of failure, and an even more remarkable durability. Now, given the choice between dysregulation and regulation, it may be time to consider the alternative hypothesis—regulation instead of dysregulation—and seriously consider the possibility that some sepsis phenotypes represent regulated functional responses.”
In other words, the physiologic pathways in sepsis have been shaped by natural selection. They are regulated in a way that tends to be adaptive when the host confronts a life-threatening infection. Importantly, these pathways evolved in an environment without ICUs or antibiotics. Even so, we do not seem to be able to improve on our body’s evolutionarily conserved responses to sepsis using targeted immune modulating drugs.
Also, as PULMCCM recently wrote on Substack, the other lesson from Wu et al.’s study is the need to be suspicious of single center trial results in sepsis. There were 19 small studies in China that pointed to a powerful benefit of thymosin α1. This was a mirage, and it is not just a Chinese problem. A litany of single center trials in sepsis have been overturned by results from better designed multicenter RCTs, including the famous Rivers trial of early goal directed therapy in sepsis. We should be especially skeptical of single center sepsis trials that show improbably large beneficial effects.
Copyright © Joe Alcock MD
Categories: Uncategorized
Joe Alcock
Emergency Physician, Educator, Researcher, interested in the microbiome, evolution, and medicine
Leave a comment