Posing “why” questions is a classic approach in evolutionary medicine. I have been interested in why oxygen regulates the immune system since medical school when I learned about diving and hyperbaric medicine and high altitude medicine. For both, oxygen treatment plays a key role.
High altitude illnesses share many of the same pathways seen in sepsis. The following graphic shows commonalities between the most severe manifestations of high altitude illnesses and sepsis, from a talk I gave at a wilderness medicine conference:
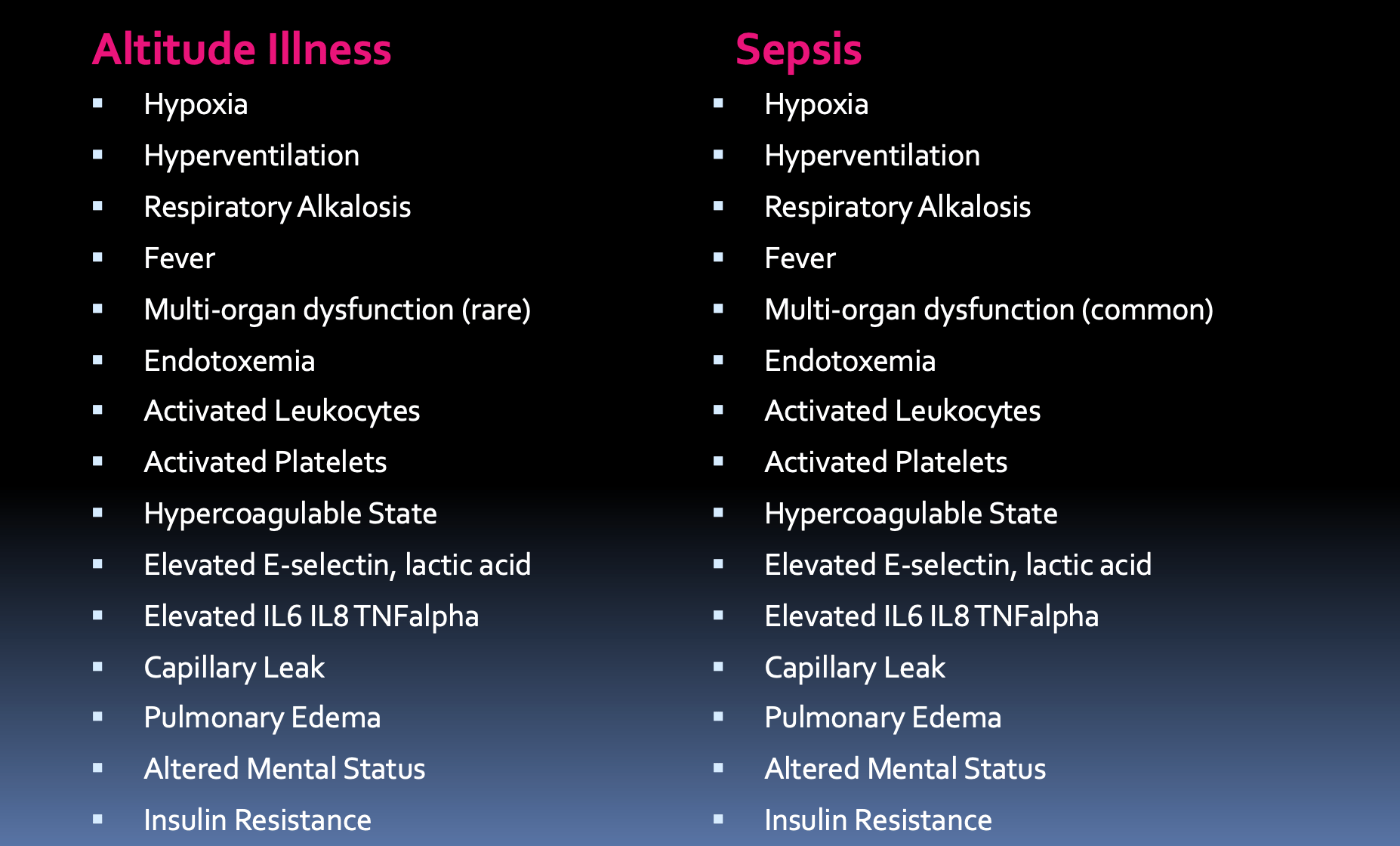
If all one had was a blood sample, you might be hard pressed to tell the difference. High altitude illnesses resemble the hyper-inflammation of sepsis. The culprit of acute mountain sickness is the relative scarcity of oxygen molecules at high altitude. Hypoxia triggers inflammation via hypoxia inducible factors. Hypoxia inducible factors (HIF) are transcription factors that regulate cellular responses to hypoxia. During hypoxia, HIF-1α along with pro-inflammatory nuclear factor kappa B both enter the cell nucleus and activate the transcription of genes involved in inflammation and metabolism. But why should these resemble sepsis? The answer may be that hypoxia alters the relationship between hosts and microbes.
Hypoxia induces changes to the gut that increase endotoxin in the bloodstream. Exercising under hypoxic conditions can lead to leaky gut, resulting in the passage of bacteria and endotoxin through the intestinal barrier. These bacterial products are recognized by TLR and nuclear factor kappa B and also by HIF 1 alpha, triggering an inflammatory cascade. These responses may have evolved to resist bacterial invasion and compensate for hypoxia’s effect on resistance to infection.
Low oxygen tension signals a heightened risk of bacterial invasion. In addition to causing leaky gut, hypoxia is an independent risk factor for infection. The mechanism involves interference with neutrophil ability to kill bacteria. Oxygen is a key substrate in bactericidal killing by neutrophils via the NADPH oxidative burst. Hypoxia impairs oxidative killing of Staphylococcus aureus. This mechanism has implications for people with chronic hypoxia. Patients with sleep apnea – who are exposed to nocturnal hypoxia – have an elevated risk of infection overall, and more pneumonia in particular.
HIF activation compensates for immune deficits in hypoxia.- but it is incomplete and comes with costs to the host. As McGovern and colleagues wrote:
“Studies involving the manipulation of cellular HIF-1α levels do not fully recapitulate the potential for lack of molecular oxygen to influence phagocyte function directly, particularly with regard to the ability to mount an oxidative burst.”
Some researchers report that HIF-1α itself can cause mortality during infections. In a mouse model of infection, under acute hypoxia, S. aureus and S. pneumoniae infections rapidly caused death, which the investigators attributed to HIF activation.

However, pre-conditioning with 7 days hypoxia protected infected animals from mortality. This improved survival was attributed to altered leukocyte metabolism. (the red dashed line shows pre-conditioned animals in the graph below.) Those results highlight that HIF has costs and benefits that depend on the presence of infection and the duration of exposure to hypoxia.
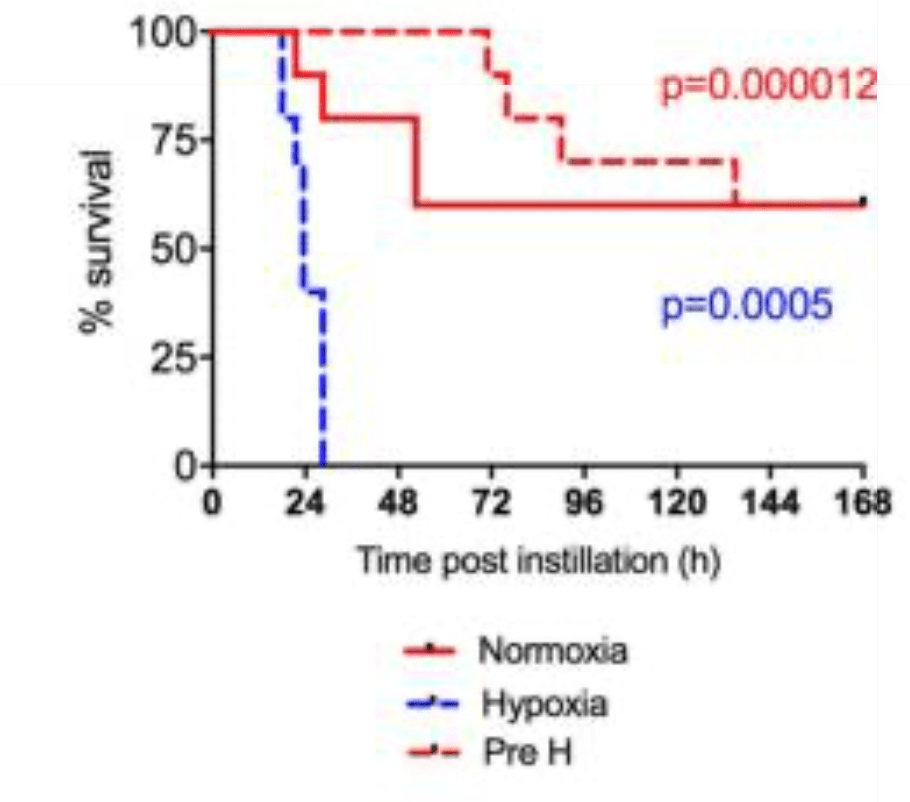
In summary, hypoxia is a risk factor for infections. HIF-1α may compensate for heightened risk of infection. HIF activation promotes bacterial clearance, speeds tissue repair, and protects the host (though not without costs) when oxygen is scarce.
Sidebar: there are a few additional wrinkles to this story:
- Pathogens vary in their responses to hypoxia. For some infections, hypoxia can protect the host, particularly aerobic Mycobacterium tuberculosis. Hypobaric hypoxia is the newly recognized reason why TB sanataria were often located at high altitude.
- Pathogens can adapt to hypoxia, for example in Pseudomonas aeruginosa infections.
- Neutrophils themselves cause hypoxia. Neutrophilic infiltration of abscesses induces ‘inflammatory hypoxia’. Neutrophils deplete local epithelial oxygen levels by consuming oxygen to fuel the oxidative burst, probably in competition with oxygen use by rapidly dividing bacteria.
- Competition for limited resources, including oxygen, between hosts and pathogens can have a counterintuitive benefit to the host if it harms pathogens and infected cells disproportionately. This may be an example of an adaptive strategy we termed immune brinkmanship.
- It is important to state that HIF did not evolve simply to provide protection from infection. HIF regulates healthy physiology, development, and also plays a role in many pathological conditions apart from infection, including for example cancer and vasculitis. This extensive repertoire needs to be taken into consideration when weighing its fitness effects.
Categories: Uncategorized
Joe Alcock
Emergency Physician, Educator, Researcher, interested in the microbiome, evolution, and medicine
1 reply ›